Nom i cognoms / Name and surname
Lourdes Ibañez
Afiliació / Affiliation
HSJD-FSJD
Programa de finançament europeu en que s’enmarca aquest projecte? / European funding programme in which this project is being carried out?
EU support (FP7, H2020, etc.) other than MSCA
Títol del projecte / Project title
PCOS IN ADOLESCENT GIRLS AND YOUNG WOMEN: TOWARD A TREATMENT GUIDED BY PATHOPHYSIOLOGY
Número del projecte / Project number
899671
Breu explicació del projecte / Brief explanation of your project
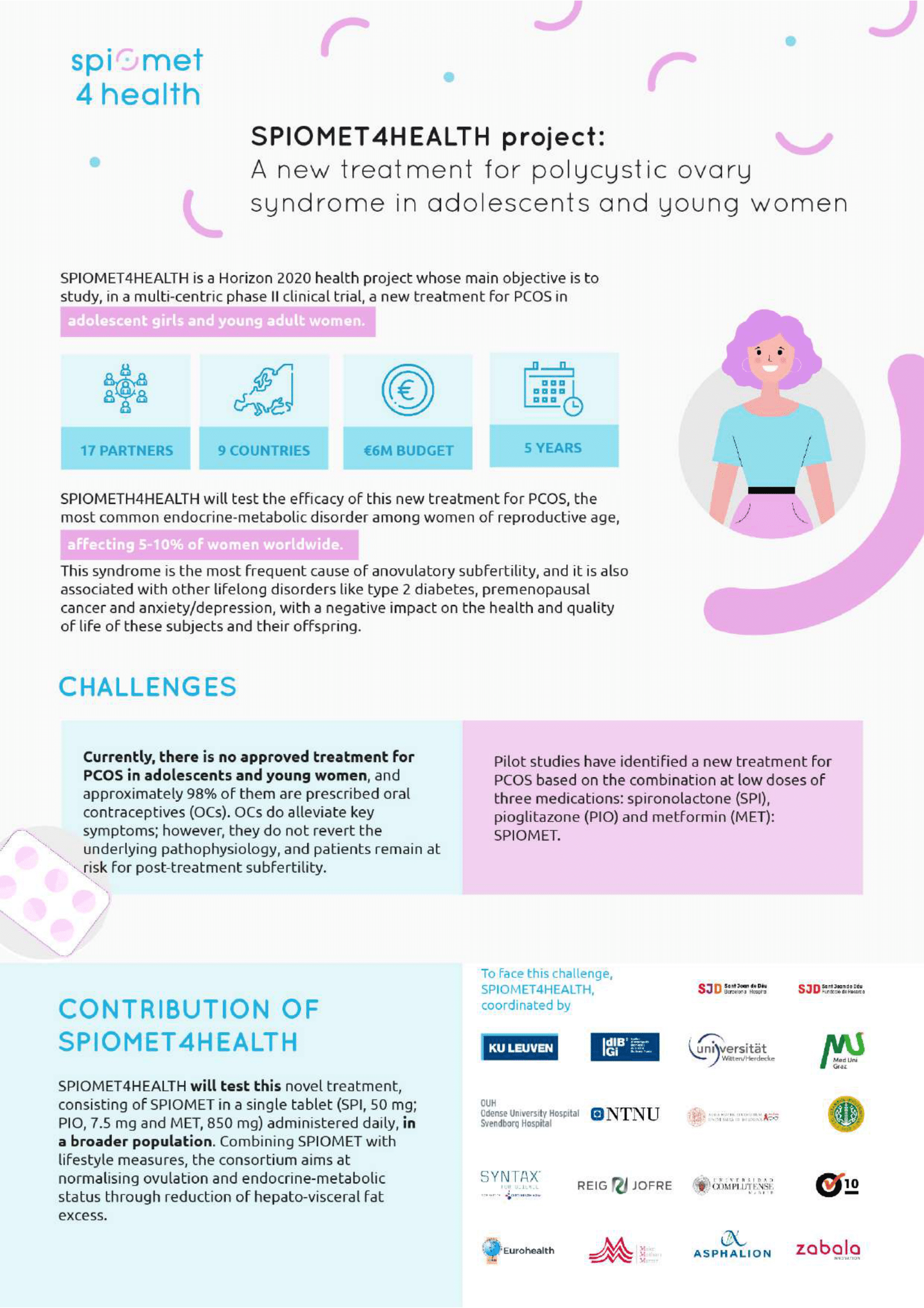
Polycystic Ovary Syndrome (PCOS) is the most prevalent chronic endocrine-metabolic disorder in women of reproductive age, affecting 5-10% of women worldwide. It is the most frequent cause of anovulatory subfertility, and it is also associated with lifelong co-morbidities such as type 2 diabetes, premature vascular aging, premenopausal cancer and anxiety/depression, with a negative impact on the health and quality of life of these subjects and their offspring.
There is currently no approved treatment for PCOS in adolescent girls and young women. Oral contraceptives (OCs) are prescribed off-label to approximately 98% of young PCOS patients, including to those without pregnancy risk. Usual care consists of treatment with an OC, followed by ovulation induction and/or costly assisted reproduction techniques. OCs do alleviate key symptoms of PCOS, such hirsutism and menstrual irregularity, but do not revert the underlying pathophysiology, and patients remain at risk for posttreatment subfertility.
Pilot studies have generated new insights into the pathophysiology of PCOS, and have thus led to the development of a new approach wherein the PCOS phenotype is reverted without side effects. The novel medication is a fixed, low-dose combination of two insulin sensitisers [Pioglitazone (Pio), Metformin (Met)] and one mixed anti-androgen and anti- mineralocorticoid, (Spironolactone (Spi)] [SPIOMET]. The new treatment proved to be superior to OCs in normalising ovulation rates (after treatment discontinuation), and endocrine-metabolic status, and thus in reverting the PCOS phenotype, apparently without side effects. However, the studies performed so far have some limitations: the medications used in those studies were combinations of generics that had to be administered separately, resulting in the intake of three different tablets, which has potential compliance problems. Moreover, those studies were unblinded, and were composed of small cohorts with limited ethnic variability so that the conclusions are not readily or widely applicable, and included mostly non-obese PCOS patients, thus limiting the relevance of the results for obese PCOS patients who constitute approximately 50% of the PCOS population.
SPIOMET4HEALTH aims to overcome the aforementioned limitations by testing, in a multi-centre, double-blind, Phase II trial, a novel treatment consisting of SPIOMET in a single tablet (SPI, 50 mg; PIO, 7.5 mg and MET, 850 mg) administered daily, on top of lifestyle measures, in adolescent girls and young adults (AYAs) with PCOS; it aims at normalising the ovulation rate and endocrine-metabolic status via the reduction of hepato-visceral fat excess in an early phase of the disorder. This approach is expected to reduce the risk of morbidity (including subsequent anovulatory subfertility) and to improve the quality of life of these AYAs.
SPIOMET4HEALTH main objectives are to demonstrate the efficacy, tolerability and safety of SPIOMET treatment, using ovulation rate as primary outcome, and the superiority of SPIOMET against the other study arms (SPIO, PIO, placebo). The trial will be conducted in a large and ethnically diverse cohort recruited in various academic centres. The design of SPIOMET4HEALTH foresees that the patients themselves will be engaged over the entire timespan of the project, and will also contribute to the ultimate study evaluation. The project incorporates a behavioural intervention and quality of life evaluations that are expected to provide the first large-scale evidence on the psycho-social benefits of the studied treatments specifically in AYAs, where the long-term impact is potentially even higher than in older women. SPIOMET4HEALTH will also analyse and model the socio-economic impact of PCOS, including the direct and indirect costs for the health care systems and for society over the short-, mid- and long-term.
Evidence on the psycho-social impact, together with the economic modelling, will lead to conclusions that will be helpful for determining pricing and financing strategies for medicines, along with more efficient models of supply related to management of PCOS in health care systems globally. These data will be crucially helpful to develop new strategies for women’s health, and to reduce the economic burden of PCOS on health systems. SPIOMET4HEALTH will provide a solid basis for further research and development (Phase III clinical trials), and ultimately for commercialisation and exploitation of SPIOMET for PCOS.
Enllaç a la pàgina web del projecte / Link to your project website
https://spiomet4health.eu/
Repte en que s’emmarca aquest projecte / Challenge within the framework of this project
1. Health, demographic change and wellbeing