Nom i cognoms / Name and surname
Stefanos Chaitoglou
Afiliació / Affiliation
Department of Applied Physics, University of Barcelona
Programa de finançament europeu en que s’enmarca aquest projecte? / European funding programme in which this project is being carried out?
Marie Skłodowska-Curie Schemes
Títol del projecte / Project title
CarboCat/ Transition metal carbides/3D graphene nanostructures for enhanced electrocatalytic hydrogen production
Número del projecte / Project number
801370
Breu explicació del projecte / Brief explanation of your project
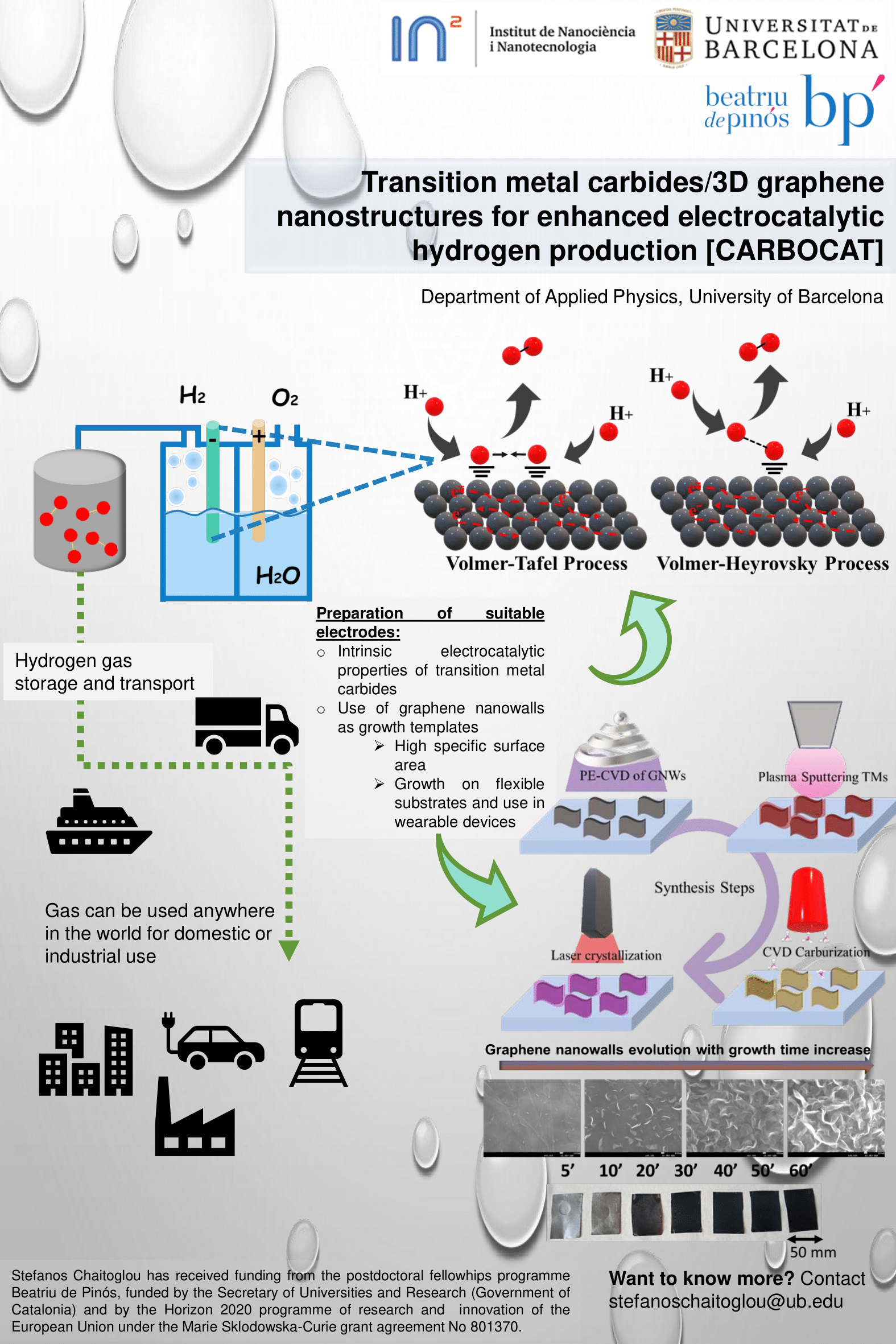
The exhaustible nature of fossil fuels places our society in seek for alternative and renewable energy carriers. Hydrogen has attracted significant attention for the above purpose, as it is the most abundant resource in the universe, while it holds the highest specific energy density of any known fuel. In addition, hydrogen is a clean fuel that, when consumed in a fuel cell, produces only water, electricity, and heat. Nowadays, H2 is mainly produced by i) natural gas steam reforming and ii) methanol reforming. Nevertheless, the first route does not consist of a renewable energy source, while methane reforming results in the production of CO2, which contributes to the greenhouse effect. Thus, the above routes are not sufficient to meet the energy demands of a post fossil fuel driven society while preventing global warming. Water splitting through electrolysis (2H2O → 2H2 + O2) is an environmentally responsible, carbon-free alternative technique for hydrogen generation. Water splitting takes place in an electrolytic cell (or electrolyzer) and requires a potential difference of 1.23 volts to occur. The hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) occur at the cathode and the anode of the cell, producing gaseous hydrogen and oxygen molecules, respectively. Heterogeneous electrocatalysis is a process that can accelerate these electrochemical reactions on the surface of catalysts materials, promoting their initialization in lower potentials and under higher rates. For the production of H2, the design and development of efficient catalysts towards the HER is of fundamental importance.
Up today, noble metals of the platinum group (e.g. Rh, Pt, Ru) are the most attractive electrocatalysts for HER. Nevertheless, the high cost and scarcity of these materials limit their potential applications. Earth-abundant transition metals (TM) based catalysts also show great potential for the HER. Especially transition metal carbides (TMC) are very promising materials for this application, thanks to their performance and availability.
Considering water electrolysis, the occurring reactions take place on the surface of the catalytic materials. Therefore, in order to increase H2 generation per electrode surface area, it is beneficial to engineer catalysts with high active surface area (offering an increased amount of active sites). The present project is prepared placing this necessity in its core and aims towards the design of novel nanostructured TMCs which can exhibit a very efficient activity towards the HER. To address this challenge, we propose a novel synthetic approach which promotes the preparation of nano-engineered TMCs films standing on graphene-based highly conductive templates that exhibit very high active surface area.
Enllaç a la pàgina web del projecte / Link to your project website
–
Repte en que s’emmarca aquest projecte / Challenge within the framework of this project
3. Secure, clean and efficient energy